If the State Agency of Medicines is asked to assess the harm of vaccines, it will have a conflict of interest, but the Ministry of Health is not deterred
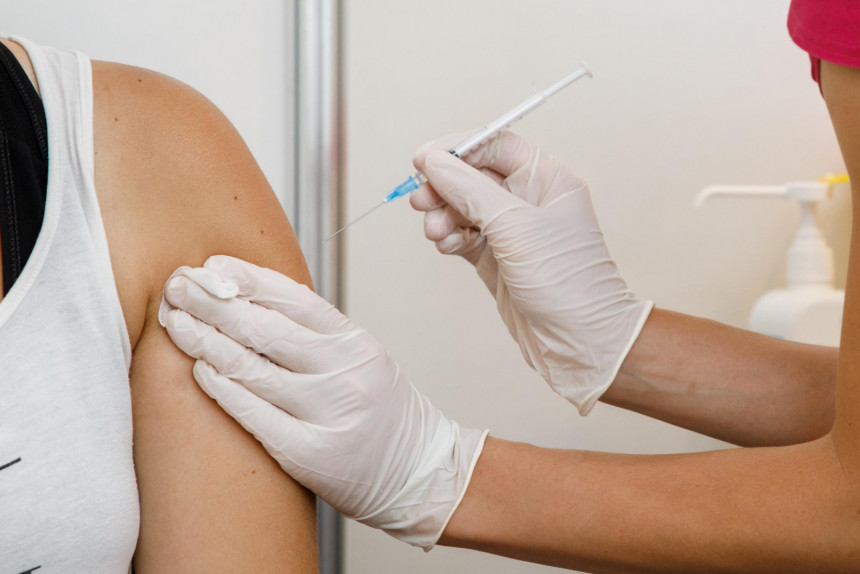
People who suffer moderate or severe health problems after being vaccinated against Covid-19 will be able to claim compensation. The Ministry of Health expects that the rules on how to apply for this compensation and how it will be assessed will be ready for consideration by the government early next year.
Despite the objection of the State Agency of Medicines, the Ministry of Health will not abandon the idea of entrusting this rather complex task to this subordinate body. The European Medicines Agency sees a significant conflict of interest in this step if the State Agency of Medicines of Latvia were to carry out the assessment of the damage to the patient's health and the amount of compensation.
Vaccine damage will be compensated
Latvian citizens will be compensated for moderate and severe complications following the Covid-19 vaccine, as laid down in the Law on the Management of the Spread of COVID-19 Infection. The law states that Covid-19 vaccinees who suffer moderate complications or severe harm from receiving the vaccine will be compensated. The law does not explain in any way what is meant by moderate; this will most likely have to be defined in the procedure for granting this compensation, which is being developed by the Ministry of Health. The Ministry of Health is currently drafting the rules and could submit them to the government for consideration in January next year.
The responsibility for assessing applications from the public and determining the amount of compensation is to be given to the State Agency of Medicines, which has publicly expressed concerns about the validity of such a decision and has also pointed to a potential conflict of interest if the body assessing the safety of medicines were also assessing patients' eligibility for compensation.
Will a conflict of interest arise?
The State Agency of Medicines has sent a letter from the European Medicines Agency to the Ministry of Health, expressing its opinion on the conflict of interest if Latvian drug safety monitors were to carry out the assessment of patient harm and the determination of reimbursement in the context of vaccination against Covid-19. "We have pointed out that drug safety monitoring is not directly related to the assessment of patient harm and the functions should be transferred to different authorities," explains Dita Okmane, a specialist at the State Agency of Medicines. The experts of the State Agency of Medicines work with the European Medicines Agency documents and data systems, so Latvian experts also have the status of European experts and are therefore bound by European obligations and rules, including on conflict of interest.
Why would the European Medicines Agency see a conflict of interest in having the State Agency of Medicines directly assess vaccine harm and compensation? The State Agency of Medicines is obliged to assess the safety and side-effects of medicines with the sole aim of ensuring that medicines are safe and, where necessary, to update the instructions for use and the descriptions.
As the body that monitors the safety of medicines and assesses adverse reaction reports, it should not, however, assess the harm to the health of an individual patient and determine the amount and payment of compensation, since this would no longer ensure that the safety assessment of medicines, i.e., vaccines, is independent and impartial in Latvia.
Dita Okmane also points out that the State Agency of Medicines does not have the expertise and experience to be able to assess the harm to a patient's health according to the criteria of the Treatment Risk Fund.
Ministry of Health: it's decided and that's how it will be
In other cases, when a patient asks for an assessment of the damage caused to his or her health during the treatment process, this is assessed by experts at the Health Inspectorate, with compensation, if any, paid by the National Health Service. When asked by Neatkarīgā why the Health Inspectorate could not also assess the potential harm caused by the Covid-19 vaccine, the Ministry of Health cites as the main argument that the State Agency of Medicines is already working on an assessment of the side effects of this vaccine.
The Ministry of Health's explanation shows that the issue is effectively settled: establishing the fact of damage to a person's health, determining compensation, paying compensation is a new function for the health sector and is delegated to the State Agency of Medicines. Oskars Šneiders, a specialist at the Ministry of Health, expressing the Ministry's opinion, told Neatkarīgā that the activities of the State Agency of Medicines are closely linked to the investigation of adverse drug reactions, which is its direct function and competence, and the Agency already carries out one stage of the entire assessment of harm. It is clear from the Ministry of Health's reply that, even despite the objection of the State Agency of Medicines, the Agency will have to do the work that the Ministry of Health imposes. Of course, the rules have yet to be agreed by the Cabinet of Ministers and it is difficult to predict at this stage whether the government will pay attention to the concerns raised about the conflict of interest.
The rules will be in government in January but will require a transitional period
Citizens who feel that their health has been moderately or severely harmed by the Covid-19 vaccine can claim compensation, but there is as yet no document describing the process - where to go, what to submit, etc. The law only states that this is a right and that an application can be made within two years of vaccination.
Oskars Šneiders, a spokesperson for the Ministry of Health, said that the Ministry is currently working with experts on draft Cabinet Regulations on the procedure for granting and paying compensation. Discussions are still ongoing to find the optimal solution for assessing the side-effects caused by the Covid-19 vaccine and to establish a compensation scheme. "We are aware that the State Agency of Medicines needs a number of new posts and specialists to perform this function, as well as additional funding for remuneration and material and technical support," says O. Šneiders. The Ministry of Health is ready to provide this support by involving other institutions.
The rules are likely to be ready in January and, if the government approves them, citizens will be able to submit claims for compensation next year. However, there is likely to be a transitional period of a few months to allow authorities to prepare for the assessment of the claim.
Is the work of the State Agency of Medicines unappreciated?
The number of reports of suspected adverse reactions to medicinal products received by the State Agency of Medicines has increased tenfold in the last year. In 2021, 3,000 adverse reaction reports have been received for Covid-19 vaccines alone. The Agency has repeatedly asked the Ministry of Health to create additional posts for pharmacovigilance (safety assessment of medicines and vaccines) functions, but at present, this is still being done with overstretched internal resources. For example, in the medicines agencies of Denmark, Norway and other countries, the number of additional staff recruited to deal with adverse reaction reports and conduct drug evaluations has increased many-fold during the pandemic.
"All the responsibilities that the State Agency of Medicines has had to deal with during the pandemic are not limited to assessing adverse reaction reports," says Dita Okmane, a specialist at the State Agency of Medicines. The State Agency of Medicines is obliged to evaluate all medicines and vaccines against Covid-19 according to the strict quality, safety and efficacy requirements of the European Medicines Agency procedures, as the registration and safety monitoring of medicines in the EU is a fully unified international process. For example, when it comes to Covid-19 vaccines, it is no longer just a few meetings a month, but extraordinary meetings where safety signals about adverse reactions to Covid-19 vaccines are continuously analyzed, recommendations are adopted, information documents are prepared, both for the public and specifically for medical professionals - letters to healthcare professionals.
In addition to the current pandemic topic, other medicines are continuously being registered, re-registered and subject to changes to ensure that the medicines available on the market are safe and effective. For example, by December 20, 2021, the Agency had registered 215 medicines and re-registered 150 medicines, including through the European common procedures for the international registration of medicines, when the Latvian registration came into force immediately in several countries of the European Union.
*****
Be the first to read interesting news from Latvia and the world by joining our Telegram and Signal channels.
